Abstract
BACKGROUND: While many older patients (pts) with AML attain complete remission (CR) after treatment (Tx) with IC, ~80% will relapse and overall survival (OS) is poor. The randomized, placebo (PBO)-controlled, phase 3 QUAZAR AML-001 trial assessed Oral-AZA (CC-486), a hypomethylating agent, in pts with AML in remission after IC who were not eligible for stem cell transplant. At the primary data cutoff in July 2019, Oral-AZA was associated with significantly prolonged OS vs. PBO: 24.7 vs. 14.8 months (mo), respectively (P < 0.001) (Wei, 2020), but the tails of the Kaplan-Meier OS curves for Oral-AZA and PBO began to converge during later time-points (after ~48 mo). More than one-quarter of all randomized pts (125/472 [26.5%]) were either still receiving Tx with Oral-AZA (n = 45) or PBO (n = 26) or remained alive in survival follow-up (n = 26 and n = 28) at the primary cutoff. Upon trial unblinding, pts continued to be followed for OS (but not relapse-free survival). We assessed longer-term OS for pts in QUAZAR AML-001 as of September 2020, after > 1 year of additional follow-up.
METHODS: Pt eligibility and study design have been reported in detail. Briefly, eligible pts were aged ≥55 years with newly diagnosed AML, intermediate- or poor-risk cytogenetics at AML diagnosis (Dx), and ECOG PS ≤3, and had achieved first CR or CRi after IC (induction ± consolidation) before screening. Within 4 mo after CR/CRi, pts were randomized 1:1 to Oral-AZA 300 mg or PBO QD for 14 days/28-day Tx cycle. After trial unblinding in July 2019, pts in the Oral-AZA arm could continue to receive Tx in an extension phase if they continued to benefit; pts in the PBO arm had Tx discontinued and were followed for OS. Kaplan-Meier estimated OS was calculated from the time of randomization until death, withdrawal of consent, or loss to follow-up, and compared between Tx arms by log-rank test. To determine whether OS was influenced by pt-related factors, we compared baseline (BL) demographic and disease characteristics of pts who were alive (on-Tx and/or in survival follow-up) for ≥ 3 years from randomization ("Long-term [LT] Survivors") vs. those of pts who died or were censored before 3 years.
Results: In all, 472 pts were randomized to Oral-AZA (n = 238) or PBO (n = 234). Median age was 68 years (range 55-86), 91% of pts had de novo AML, and 86% had intermediate-risk cytogenetics. Upon trial unblinding, 39 pts (16%) in the Oral-AZA arm continued into the extension phase Overall, 31.4% and 15.5% of pts received > 24 mo of Tx with Oral-AZA or PBO, respectively.
At the updated follow-up in September 2020, 54 pts (23%) in the Oral-AZA arm were alive in survival follow-up, including 31 pts (13%) still receiving Oral-AZA in the extension phase; 165 pts (69%) had died and 19 pts (8%) had withdrawn consent or were lost to follow-up. In the PBO arm, 35 pts (15%) remained alive, 176 (75%) had died, and 23 (10%) had withdrawn consent or were lost to follow-up.
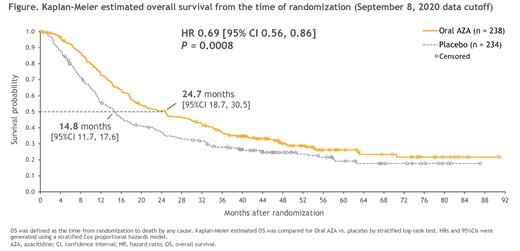
At a median follow-up of 51.7 mo, median OS in each arm remained unchanged from the primary cutoff date: 24.7 vs. 14.8 mo with Oral-AZA vs. PBO, respectively (P = 0.0008); however, the KM OS curves for Oral-AZA and PBO showed greater separation with additional follow-up, and the two curves did not touch or cross at any time (Figure). KM-estimated 3-year survival rates were 37.4% vs. 27.9% in the Oral-AZA and PBO arms, respectively (∆ +9.5% [95% CI 0.9%, 18.1%]).
The LT Survivors cohort comprised 140 pts (29.7%) in the Oral-AZA (n = 83) and PBO (n = 57) arms who were known to be alive for ≥ 3 years. Compared with pts who died or were censored before 3 years, those in the LT Survivors group were more likely to have intermediate-risk cytogenetics (95% vs. 82%) and an NPM1 mutation (45% vs. 9%) at AML Dx, and less likely to be MRD+ at BL (33% vs. 52%). Among pts with post-IC MRD+ at BL, 71% (34/48) in the LT Survivors cohort achieved MRD negativity on-study, compared with 15% (26/172) in the < 3-year cohort (P < 0.0001).
Conclusions: With > 1 year of additional survival follow-up, median OS in QUAZAR AML-001 remained unchanged in both Tx arms, but the tails of the Oral-AZA and PBO OS curves showed greater separation at later time-points than in the primary analysis (which may have been confounded by extensive censoring), indicating a sustained, long-term OS benefit with Oral-AZA. Intermediate-risk cytogenetics and NPM1 mutations at AML Dx, and absence of detectable MRD post-IC, were associated with long-term survival in QUAZAR AML-001.
Wei: Novartis, Celgene, AbbVie, Servier, AstraZeneca, and Amgen: Research Funding; Walter and Eliza Hall Institute: Ended employment in the past 24 months; Novartis, Astellas, Pfizer, MacroGenics, AbbVie, Genentech, Servier, Celgene, Amgen, AstraZeneca, Janssen: Honoraria. Döhner: Pfizer: Research Funding; Oxford Biomedicals: Honoraria; Novartis: Honoraria, Research Funding; Janssen: Honoraria; Jazz: Honoraria, Research Funding; Helsinn: Honoraria; GEMoaB: Honoraria; Celgene: Honoraria, Research Funding; BMS: Honoraria, Research Funding; AstraZeneca: Honoraria; Berlin-Chemie: Honoraria; Astex: Honoraria; Astellas: Honoraria, Research Funding; Roche: Honoraria; Amgen: Honoraria, Research Funding; Abbvie: Honoraria, Research Funding; Agios: Honoraria, Research Funding. Sayar: BMS: Honoraria. Ravandi: Taiho: Honoraria, Research Funding; Xencor: Honoraria, Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Prelude: Research Funding; Syros Pharmaceuticals: Consultancy, Honoraria, Research Funding; Astex: Honoraria, Research Funding; Jazz: Honoraria, Research Funding; Agios: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; AstraZeneca: Honoraria; Novartis: Honoraria. Montesinos: Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Incyte: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Sanofi: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Stemline/Menarini: Consultancy; Forma Therapeutics: Consultancy; Glycomimetics: Consultancy; Daiichi Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Karyopharm: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Teva: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Tolero Pharmaceutical: Consultancy; Agios: Consultancy; AbbVie: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Astellas Pharma, Inc.: Consultancy, Honoraria, Other: Advisory board, Research Funding, Speakers Bureau. Dombret: Amgen: Honoraria, Research Funding; Incyte: Honoraria, Research Funding; Jazz Pharmaceuticals: Honoraria, Research Funding; Novartis: Research Funding; Pfizer: Honoraria, Research Funding; Servier: Research Funding; Abbvie: Honoraria; BMS-Celgene: Honoraria; Daiichi Sankyo: Honoraria. Selleslag: Novartis: Consultancy, Honoraria, Speakers Bureau; Celgene: Consultancy, Honoraria, Speakers Bureau; Amgen: Consultancy, Honoraria, Speakers Bureau; Janssen Cilag: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria, Speakers Bureau; Alexion: Consultancy, Honoraria; GlaxoSmithKline: Consultancy, Honoraria; Merck: Consultancy, Honoraria, Speakers Bureau; Pfizer: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria, Speakers Bureau; Takeda: Consultancy, Honoraria, Speakers Bureau; Incyte: Consultancy, Honoraria; Teva: Consultancy, Honoraria. Skikne: Bristol Myers Squibb: Current Employment. Beach: Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Tian: Bristol Myers Squibb: Current Employment. Roboz: Celgene: Consultancy; MEI Pharma - IDMC Chair: Consultancy; Astellas: Consultancy; Jazz: Consultancy; Amgen: Consultancy; Mesoblast: Consultancy; Agios: Consultancy; Novartis: Consultancy; Otsuka: Consultancy; Janssen: Consultancy; AbbVie: Consultancy; Daiichi Sankyo: Consultancy; Helsinn: Consultancy; AstraZeneca: Consultancy; Bayer: Consultancy; Actinium: Consultancy; Astex: Consultancy; Glaxo SmithKline: Consultancy; Bristol Myers Squibb: Consultancy; Blueprint Medicines: Consultancy; Jasper Therapeutics: Consultancy; Janssen: Research Funding; Pfizer: Consultancy; Roche/Genentech: Consultancy.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract